Thinking@TheCulture is a series of questions that we, as tutors feel that are useful in helping students think and improve their understanding.
Thinking Math@TheCulture is curated by KS. More of him can be found here
This is a very interesting vectors question from a recent JC BT
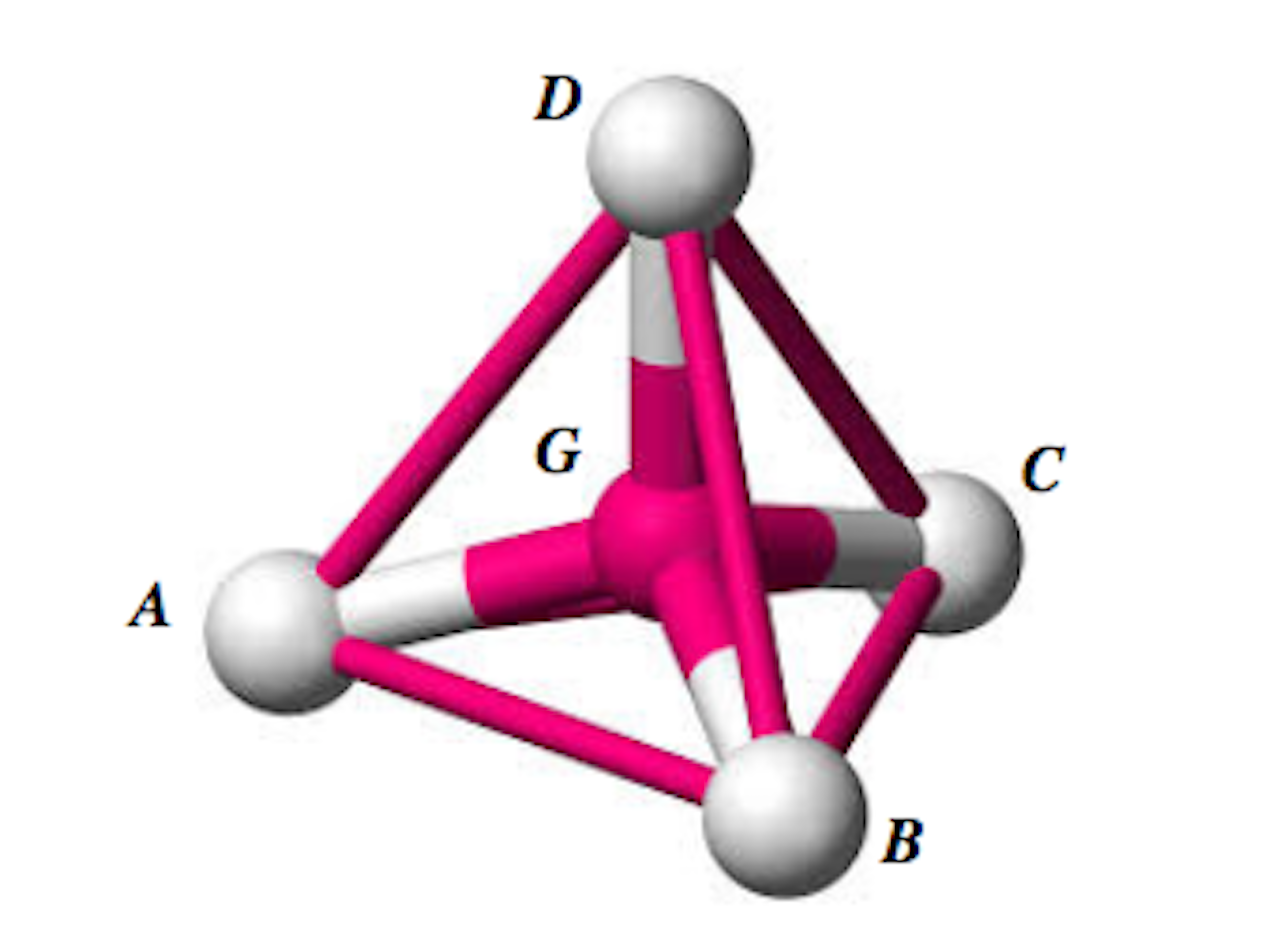
Shown in the diagram is a methane molecule consisting of a carbon atom, G, with four hydrogen atoms, A, B, C, and D, symmetrically placed around it in three dimensions, such that the four hydrogen atoms form the vertices of a regular tetrahedron.
Treat A, B, C, D, and G as points. The coordinates of A, B, C, and D are given by (5, -2, 5), (5, 4, -1), (-1, -2, -1) and (-1, 4, 5) respectively, By considering the line DG and the symmetrical properties of methane, find the bond angle of methane, that is, ![]() .
.