All solutions here are SUGGESTED. Mr. Lee will hold no liability for any errors. Comments are entirely personal opinions.
[Please do not ask me how many marks for A. The bell curve is something that is out of your control so there is no point in estimating your grade based on the number of marks you have lost. No one can tell you any accurate information on this. I hope you can learn from your mistakes here and do not make the same mistakes in Paper 1.]
Question 1
1 (a) (i) Amount of menthol = 1.32 x 10-2 x 2 = 0.0264 mol
Mass of menthol = 0.0264 mol x (10 x 12 + 20 + 16)
= 4.1184 g
Percentage mass = 4.1184 / 10.0 x 100%
= 41.2% (3 sf)
(ii) 3 chiral centres, 23 = 8 optical isomers
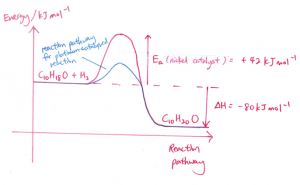
(b) (i)
(ii)
(iii) The catalyst is in a solid state and it functions as a heterogeneous catalyst as it is in a different phase than menthone (liquid state) and hydrogen (gaseous state). Menthone and hydrogen will undergo adsorption at the active sites of the surface of the catalyst, forming weak bonds between the reactant molecules and the catalyst and this causes the bonds in menthone and hydrogen to be weakened. This provides an alternative pathway which has a lower activation energy. The reactant molecules are now in closer proximity with one another and hence, the frequency of effective collisions between menthone and hydrogen gas increases and this increases the speed of reaction.
(iv) Iron might be suitable as a catalyst. Iron is a transition metal with incompletely filled 3d orbitals.
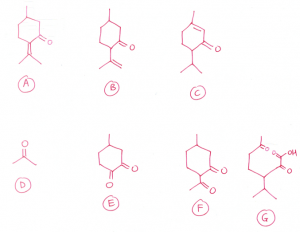
(c) All three isomers undergo electrophilic addition with bromine water. Hence, they contain carbon-carbon double bond.
All three isomers contain carbonyl group as they undergo condensation reaction with 2,4-dinitrophenylhydrazine.
All three isomers do not contain aliphatic aldehyde group.
All three isomers undergo reduction with hydrogen gas to form menthol with molecular formula C10H20O. Hence, all three isomers contain two functional groups that can be reduced by hydrogen gas as 4 hydrogen atoms are added.
Menthol contains a secondary alcohol, hence, the 3 isomers contain a ketone group. The other functional group that is reduced must be carbon-carbon double bond.
When reacted with hot concentrated KMnO4, strong oxidation or oxidative cleavage occurs.
A gives D, which is a ketone, and E which contains two ketone groups (one ketone group is originally present in A).
B gives F, which contains two ketone groups (one ketone group is originally present in B). B contains a terminal carbon-carbon double bond.
C contains a carbon-carbon double bond in a ring. G contains a carboxylic acid group and two ketone groups (one ketone group is originally present in C).
D, F and G contains CH3C=O group as they undergo positive iodoform test (oxidation), forming yellow precipitate of CHI3.
Question 2
(a) The volatilities of the halogens decrease from chlorine to iodine (i.e. melting and boiling point increase from chlorine to iodine).
From chlorine to iodine, the number of electrons increases. Hence the size of the electron cloud increases which increases the strength of van der Waals’ forces of attraction between the halogen molecules. Hence, the volatilities decrease from chlorine to iodine.
(b) 3Cl2(g) + 6OH–(aq) → 5Cl–(aq) + ClO3–(aq) + 3H2O(l)
The oxidation number of chlorine increases from 0 in Cl2 to +5 in ClO3–
The oxidation number of chlorine decreases from 0 in Cl2 to -1 in Cl–
(c) (i) The bond energy for H–F is 562 kJ mol-1 while the bond energy for H–Cl to H–I decreases from 431 to 366 to 299 kJ mol-1. H–F bond has the highest bond energy so the bond strength is the strongest which require the most energy to break. Hence, HF does not dissociate completely in water to produce hydrogen ions.
(ii) pH of HCl = – lg (0.50) = 0.301
Ka = [H+]2 / [HF] [H+]2 = 5.6 x 10-4 x 0.50 = 2.8 x 10-4
pH = – lg [(2.8 x 10-4)1/2] = 1.78
(d) When aqueous silver nitrate is added to chloride ions, a white precipitate of silver chloride (AgCl) is formed.
Ag+(aq) + Cl–(aq) → AgCl(s)
When aqueous ammonia is added to silver chloride, the white precipitate dissolves to form a colourless solution of [Ag(NH3)2]+
AgCl(s) ⇌ Ag+(aq) + Cl–(aq)
Ag+(aq) + 2NH3(aq) → [Ag(NH3)2]+
When aqueous silver nitrate is added to iodide ions, a yellow precipitate of silver iodide (AgI) is formed.
Ag+(aq) + l–(aq) → Agl(s)
When aqueous ammonia is added to silver iodide, the yellow precipitate is insoluble in aqueous ammonia.
(e) (i) The value of pV remains constant.
(ii) pV = nRT
12.0 x 105 x V = 0.40 x 8.31 x 300
V = 8.31 x 10-4 m3
(iii) 9.26 x 105
8.88 x 105
pV = 9.11 x 105 Pa dm3
V = 0.759 dm3
(iv) There is presence of permanent dipole-permanent dipole interactions between hydrogen chloride molecules due to the net dipole moment present as the chlorine atom is electronegative. These intermolecular forces of attraction are significant and will deviate from the ideal gas properties. Hence, the molecules are closer together and occupy a smaller volume as compared to an ideal gas.
(f) (i) ΔG = ΔH – TΔS
0 = +16.8 – 188ΔS
ΔS = +0.089362 kJ K-1 mol-1 ≈ +0.0894 kJ K-1 mol-1 (3 sf)
The entropy change is positive because there is an increase in disorderness when the liquid state of hydrogen chloride is changed to the gaseous state as the molecules have an increased number of ways of arranging themselves in the gaseous state.
(ii) ΔG = +16.8 – (298)(0.089362) = –9.83 kJ mol-1 (3 sf)
A negative sign for ΔG means that the reaction is feasible and can take place spontaneously.
Question 3
(a) (i) Magnesium burns with a brilliant white flame when it reacts with oxygen to form magnesium oxide.
2Mg(s) + O2(g) → 2MgO(s)
Calcium burns with a brick-red flame when it reacts with oxygen to form calcium oxide.
2Ca(s) + O2(g) → 2CaO(s)
(ii) Magnesium oxide is sparingly soluble in water, forming magnesium hydroxide while calcium oxide is partially soluble in water, forming aqueous calcium hydroxide which is alkaline in water.
MgO(s) + H2O(l) → Mg(OH)2(aq)
CaO(s) + H2O(l) → Ca(OH)2(aq)
(b) (i) 2.0 x 10-1 mol dm-3
(ii) Ca(OH)2 ⇌ Ca2+(aq) + 2OH–(aq)
Concentration of OH– = 2.5 x 10-2 x 2 = 5.0 x 10-2 mol dm-3
pOH = – lg (5.0 x 10-2) = 1.30
pH = 14 – 1.30 = 12.7
(iii) Ksp = [Mg2+][OH–]2
Ksp = (s) (2s)2 = (1.6 x 10-4)(3.2 x 10-4)2 = 1.64 x 10-11 mol3 dm-9
(iv) White precipitate of magnesium hydroxide would be observed. Barium hydroxide is more soluble in water than magnesium hydroxide. Hence, the concentration of hydroxide ions increases. Common ion effect occurs and hence, the solubility of magnesium hydroxide decreases so less magnesium hydroxide is able to dissolve in water.
(c) (i) Aspartate and glutamate
Ionic bonding
(ii) The alpha helix is held in place due to hydrogen bonding formed between the N–H group of each amino acid and the fourth C=O group following it along the chain.
(iii) H2SO4(aq), heat under reflux / NaOH(aq), heat under reflux
(iv) gly-asp-gly-tyr-ile-ser
(d) (i)
(ii) Step 4: Reagent: excess concentrated ethanolic NH3
Condition: heat in sealed tube
Step 5: Reagent: H2SO4(aq)
Condition: heat under reflux
Question 4
(a) Step 1: hydrolysis
Step 2: dehydration
Step 3: reduction
(b) C6H8O(l) + 15/2 O2(g) → 6CO2(g) + 4H2O(l)
Energy taken in for bond breaking
= 8 (C – H) + 3 (C – C) + 2 (C = C) + 2 (C – O) + 15/2 (O = O)
= 8(410) + 3(350) + 2(610) + 2(360) + 15/2 (496)
= 9990 kJ mol-1
Energy released for bond formation
= 12 (C = O) + 8 (O – H)
= 12(805) + 8(460)
= 13340 kJ mol-1
Enthalpy change of combustion = 9990 – 13340 = –3350 kJ mol-1
(c) Q = mcΔT = (200)(4.18)(32) = 26752 J
100% heat = 26752 / 80 x 100 = 33440 J
Amount of DMF = 1.00 / 96 = 0.01042 mol
Experimental enthalpy change of combustion
= 33.440 / 0.01042 = –3209 kJ mol-1 ≈ –3210 kJ mol-1
The enthalpy change of combustion obtained in (b) uses bond energies from the data booklet which are average values of bond energies obtained from a range of molecules containing that bond. In addition, some of the heat energy released from the burning of DMF is lost through the heating of the container itself or through the surroundings. Hence, the experimental value is less exothermic. Hence, there is a slight difference in both values.
(d) (i) R–CH2OH + H+ → R–CH2OH2+ (fast)
R–CH2OH2+ → R–CH2+ + H2O (slow)
R–CH2+ + Cl– → R–CH2Cl (fast)
The second step (R–CH2OH2+ → R–CH2+ + H2O) is the rate determining step.
(ii) Reagents: K2Cr2O7(aq), H2SO4(aq)
Condition: heat under reflux
[KMnO4(aq), H2SO4(aq) is not accepted due to oxidative cleavage]
(e) (i) Ester
(ii) HO–CH2–CH2–OH
(iii) Reagents: concentrated sulfuric acid
Condition: heat under reflux
(f) Name of mechanism: Free radical substitution
Initiation
Cl–Cl → 2 Cl·
Propagation
Cl· + R–CH3 → R–CH2· + HCl
R–CH2· + Cl2 → R–CH2Cl + Cl·
Termination
Cl· + Cl· → Cl2
R–CH2· + R–CH2· → R–CH2CH2–R
R–CH2· + Cl· → R–CH2Cl
Question 5
(a) (i) Proton number is the number of protons in the nucleus of the atom.
Nucleon number is the number of protons and neutrons in the nucleus of the atom.
(ii) Let the relative abundance of 7Li be k
Relative abundance of 6Li = 1 – k
k x 7.016 + (1 – k)(6.015) = 6.942
7.016k + 6.015 – 6.015k = 6.942
1.001k + 6.015 = 6.942
1.001k = 0.927
k = 0.9261
Therefore, relative percentage abundance of 7Li = 92.61%
Relative percentage abundance of 6Li = 7.39%
(iii) X = 3He Y = 7Li
(b) (i) Ionic bonding. The lithium atom is able to transfer its valence electron to the carbon atom in graphite which has one unpaired electron, forming positive Li+ cation and anionic graphite. The bonding between the positive Li+ cation and anionic graphite is ionic. [answer is edited]
(ii) Before discharge: +4
After the cell is totally discharged: +3
(iii) The shape is tetrahedral with respect to boron. There are 4 bond pairs of electrons and 0 lone pair of electrons for boron. Hence, the shape is tetrahedral.
(iv) Cold, alkaline KMnO4(aq)
(v) Condensation
(c) (i) 2Li2O2 + 2CO2 → 2Li2CO3 + O2
(ii) Lithium has similar chemical properties as magnesium because the atomic radius and electronegativity are similar. Lithium is smallest in size for Group I metal and hence, lithium ion has the largest charge density for Group I metal ions. Therefore, it is able to polarise and distort the electron cloud of the carbonate anion to a larger extent and the distorted electron cloud of the carbonate anion is more readily decomposed by heat energy. Hence, the thermal stability of lithium carbonate is low and can be easily decomposed.
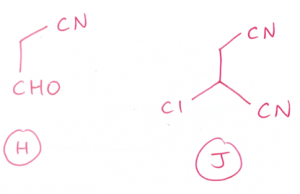
(d) (i) CH3CH2CH2CHO + CH3CH2Br
OR
CH3CH2CHO + CH3CH2CH2Br
(ii) (CH3CH2)2CO + CH3Br
OR
CH3CH2COCH3 + CH3CH2Br
(e) Only P and Q will turn orange acidified potassium dichromate(VI) to green. R, being a tertiary alcohol will not be oxidised and will not turn orange K2Cr2O7 to green.
After oxidation, 2,4-dinitrophenylhydrazine can be added. For Q, after oxidation, the product is a ketone and will form an orange precipitate with 2,4-dinitrophenylhydrazine. For P, after oxidation, the product is a carboxylic acid and will not form an orange precipitate.
Please do let me know of any mistakes or typing errors that I made while rushing this. Much appreciated and thanks!
[…] Mr Eric Lee on 2015 A Level H2 Chemistry (9647) Paper 3 Suggested Answers […]
Leave a Comment


Hope the amswers would be up asap :)!!
Hi. The answers will be up tonight. I need some time to prepare the answers.
Ya, I placed the C=C for struct C… the Carbon connected the CH3 and the adjacent Carbon in the ring (but not the one next to the C=O)
It is okay
What happens if I did all 5 qns for paper 3 and submit all 5?
The first 4 questions will be marked. The last question will be ignored.
Hi if I wrote Ag(NH3)4 instead of Ag(NH3)2 with all the equations and explanations right will I get 3 marks deducted?
Some marks are deducted, not all.
Can the C=C for structure C be placed elsewhere?
The carbon-carbon double bond must be placed beside the methyl group, because G gives a positive iodoform test.
So can be on the other side of the compound? Like opposITE to what you drew
Yes, that can be accepted.
I think I did the same as ChemKid: “Ya, I placed the C=C for struct C… the Carbon connected the CH3 and the adjacent Carbon in the ring (but not the one next to the C=O)”
that would still result in a methyl ketone group right…
Yes
Isn’t it 16 chiral carbon
Nevermind, found the mistake:(
3 chiral carbon only. Chiral carbon must have 4 different groups bonded to it
hi how many marks will i lose if i didnt have time to draw the structures?
You will get some marks if the chemistry in your deductions are correct.
Just wondering, what if I didn’t draw A-G (I only had time to draw D) but wrote all the elucidation points you listed above? What’s the maximum number of marks that can be awarded for the explanations?
No one knows the maximum marks but you will get marks for the chemistry in the deduction.
is each structure half mark or one mark? (:
No half mark marking
For question 1(c), for structure C, can be alkene group be placed on the left side of the methyl group instead of the right?
Yes
Can the C=C double bond for structure B be at the very top?
No, you need to form methyl ketone for a positive iodoform test
Hi, how are the marks allocated for 1c? Is it 1m per structure, then how about the elucidation? Thanks!
The chemistry behind the deduction would carry some marks so there will be more than 10 marks for marking points. Even if you cannot draw the structures, you will be able to score some marks if your deduction is logical and correct
Hello, can the C=C for structure B be placed at the very top?
No, F must have a positive iodoform test, which means I need to have methyl ketone in the structure after oxidative cleavage. If you put the C=C at the top, there is no methyl group at the side.
Can I use Cu for ibiv
Common transition metals with incompletely filled 3d orbitals should be accepted
Anyone else left out the last page? ):
Nope.
Qn1 part a , i got caught up and didnt x2 the moles . Will there be error carry forward?
Most likely no, the question is only 2 marks
Qn1 part a , i got caught up and didnt x2 the moles . Will there be error carry forward? ..
Not likely
Can part biv be pd palladium?
Yes
I wrote palladium for the transition metal, but the reason given was that it was in the same group as nickel and platinum instead of the incompletely filled d orbitals, is that accepted? :/
No, your answer is quite vague. The key concept is about incompletely filled 3d orbitals
Hi I put palladium too but my explanation was that all the 3 elements have the same number of electrons in their d subshell, hence they will exhibit similar chemical properties. Is that possible?
The main idea is that the transition metal that you write has incompletely filled 3d orbitals
How are elucidation marks given? I only wrote what functional groups are present based on the data given but I didn’t specify the type of reaction like oxidation. 🙁 very worried now because I couldn’t elucidate the structures
For elucidation question it is important to write down functional groups deduced and he type of reaction. You may get some marks if you can deduce the correct functional groups
hey for part iv its just any of the transition elements? is manganese accepted?
Yes, manganese should be accepted.
omg i got full marks for q1
Sorry for Q1ai why is number of mol of menthol x2 instead of x1/2 of Hydrogen gas? Since 1 -OH produces 1/2 H2(g)?
Yeah , that is why.
The mol of H2 is given. So your mole ratio is that 1:0.5 . Mol of H2 x2 = mol of methol
R-OH +Na (+)-> R-O(-)Na(+) +1/2 H2(g)
oh ya shit saw it thanks
does each cmpd for the deduction carry 1 mark each?
Yes but there should be more than 10 marking points
So it is possible to earn >10 marks if structure and the chemical deductions are correct?
Very unlikely….I think they reserve 4 marks for all the structures…
My sch give half marks for each deduction though
No half marks are given.
Lol the obvious answer is no, how is it possible?!
thanks! fingers crossed that I can at least 7marks
I heard from my tuition teacher that there are “bonus marks” . Is that true?
Nope.
Hope i’m not asking you too early. For Q2d) if i got everything correct but I included the case of Br- as well (it is not asked) do I still get full marks?
Br- portion will be ignored. In A level, overall they may penalize some marks for writing irrelevant information that is not answering the question.
Can the tm be Zinc ?
Zinc has completely filled 3d orbitals. It cannot be a heterogeneous catalyst.
hi!! just to check, vanadium is also another element that is accepted for 1biv?
vanadium(V) oxide acts as a catalyst, not vanadium itself.
For 1biii because it’s a 2m qn I didn’t write the part about bring reactants to closer proximity but I wrote everything else cause I thought it was enough.. Will I be penalised?
Idea marking. Should be fine.
Hi Mr Lee
For part (i), I forgot to substitute in the value for the activation energy (42) into the graph (I just labelled it as Ea), will marks be deducted?
For part (iv), I wrote Palladium and stated that it was a TM, but explained it wrongly by saying it had energetically accessible d orbitals for bond formation, so do I still get the one mark?
For part (c), I merely stated the functional groups present based on the distinguishing tests but I didn’t specify the exact reactions occurring, do I still get marks? And will there only be a maximum of 3 marks for deductions since there are 7 structures to be elucidated and assuming each structure carries 1 mark?
Thank you!
(i) yes
(iv) the key concept should be incompletely filled 3d orbitals
(c) in elucidation, functional groups AND type of reaction must be stated. There should be more than 3 marks for deductions.
Can i write all 3 does not have aromatic aldehyde instead since it does not undergo mild oxidation with fehlings solution ?
aromatic aldehyde is tested using Tollen’s reagent, not Fehling’s
how are the marks distributed for the deductions? like I wrote 8 explanations, and only drew D. didn’t write oxidation or reduction or whatever, just the type of structures it could have contained. couldn’t think straight. great i feel like craaaaap.
Out of the 10 marks, 3 are awarded to deductions, 1-2 marks for the structures.
3 + 1/2 = 5 max though? lol
Exactly the same as me sigh
the best method to do this type of qns is to do guess and check with the reaction involved and molecular formula given. If not you will spend a lot of time thinking of the correct structure…
I have no idea why I couldn’t do the question… usually organic Chem and elucidation are my strongest parts in Chem :/ never mind, thanks for the advice anyway 🙂 good luck for ur remaining papers
Woah high five man. Sigh how did u guys find the rest of the paper ?
mindblock during exams are really common! bled a lot of marks for carelessness too. at least it’s over. yay! 😛
Hi, for the mode of action of the catalyst, it is only 2 marks, are so many points necessary??
I am writing the full answers. There may be more than 2 marking points.
Hi there, thanks for doing this 🙂 can the alt catalyst be Silver because of a diagonal relationship with Nickel?
Silver has a completely filled 4d orbital. It is not accepted.
Can I put Tin for the example ???
Tin is not under transition metal
For 1b(iii), will I be penalized if I didn’t mention the part about lowering activation energy but I mentioned everything else. Because I thought they were only asking you to describe what happens during the reaction ..
It is better to mention
hi for qn 1) a part ii i wrote 4 chiral centres and 16 optical isomers can still get 1 mark since i applied 2^n correctly?
I highly doubt you will get ecf, but no one will know the mark scheme.
hi for the energy diagram, if I labelled the -80KJ wrongly and I didn’t write the equation, how many marks out of 2 will I get?
also will the errors affect my marks for the part bii (i showed lower activation energy)?
Part II should not be affected. Marks will be deducted for the first part. You need to label correctly the delta H, Ea, reactants and products
Hi, if I didn’t draw the x axis for the reaction pathway then how many marks will I get?
probably out of 3 max 1
Hi. If I didn’t draw the x axis for the reaction pathway. How many marks will I get?
1 mark
Is palladium accepted as a catalyst?
Yes
So A to G is not 7marks?
If you get all 7 structures correct it should be 7 marks and most likely your deductions are correct as well, so 10 marks should be no problem.
The answers are taking really long today 🙁
Please have some patience while the answers are being prepared. I have other work commitments as well so please be appreciative of the effort in preparing for the answers.
dude he is providing free solutions.. isnt that kind enough already? be a bit more thankful would u
Yea he is impatient to know how bad he has done.
Why can’t it be any transition metal?
it can be any transition metal
Hello are all the answers posted? Not sure if it’s my phone I seems to only be able to look at question 1…
All the answers will be posted soon. Question 2 is ready.
For qn 2b, I wrote the cold Naoh eqn… Didn’t read carefully. Would I be penalized the whole 2 marks…
The question asks for hot NaOH, so please be more careful
So I won’t get marks for correct oxidation numbers?
for question 2 part 3, can i use other values that is between 9.26 X 10^5 and 8.88 X 10^5? i think i used 9.10 X 10^5 then got V = 0.758 dm^3
For Qn 2eiii, I estimated 9.00*10^5. Is any number between 9.26*10^5 and 8.88*10^5 acceptable?
Not sure of the acceptable range in their marking scheme, but the answer must be between 9.26 x 10^5 and 8.88 x 10^5
Any idea what the A threshold might be for this paper?
Hahhaha I think u shouldn’t be asking that cos he specifically stated above that no one should ask him about the bell curve 🙂
I think >60 for this p3 because its pretty straightforward
my friends all got >70 though, maybe it’ll be higher than 60. 60 is too low.
My delta S units was wrong , will I get penalise for that ?
It is a common mistake. Please take note
Is there Ecf for Chem
No
Hi, thank you for taking time off to work out the solutions. I would like to clarify somethings 🙂
For Q2b) is it necessary to use INCREASE and DECREASE? Could I replace it with changed with the rest of the phrasing the same?
For Q2iv), could I explain it in terms of VDW between HCl molecules being significant due to it being in low temperatures? resulting it to exhibit negative deviation, it’s still in terms of properties of HCl molecules since I explained its structure and it existing as discrete molecules, just not the polarisation part.
Thank you!
2(b) you need to state increase AND decrease because the reaction is disproportionation.
2(e)(iv) you need to mention pd-pd for HCl which are more significant intermolecular forces of attraction. The temperature is 300K, it is not low.
Hi sir, could u do an explainatiom on how you got the values for 2eiii on ” use your values of pV to calcukate V….” ?? I dont seem to understand what the question wants…
thank you!
pV must be estimated between 9.26 x 10^5 and 8.88 x 10^5. Once you estimate pV, then use this value to divide by the given p, which is 12.0 x 10^5 Pa to find the V.
Oh shoots… i thought they wanted me to use part ii calcukated V value…
Oh well….
Thank you so much for working out the solutions!!! :)))
Hi for 2b do you include the Na in the equation? Also I think I forgot to write in state symbols. Will I be penalised?
question didnt specify so no.
the question didnt ask for state symbols
you can include Na if you are writing chemical equation instead of ionic equation. State symbols should be present for ionic equations. They may not be required for chemical equations, but it is always good to write state symbols for A level.
Hi, just a quick question, is state symbols required for q2b? If I wrote cl2 as aq instead of gas will I get the mark? Thank you 🙂
State symbols may not be required if you write chemical equations, but you should write state symbols for ionic equations. Chlorine exists as a gas unless they say chlorine water or aqueous chlorine.
Hi, for Q1c, if I have most of the answer but am missing 1-2 of the deductions (I have the other deductions and the structures) will I still have marks deducted?
And also just wanted to say thank you for the answers! Really appreciate the detailed explainations
There should be more than 10 marking points, you may be able to hit 10 marks with your deductions and structures. The marking scheme is out of your control.
Hi!!! For the hydrolysis of peptide linkage, must we write reflux for several hours!!
nope i don’t think so because reflux for hours could lead to complete hydrolysis alr (question stated partial)
heat under reflux is sufficient
Is heat for several hours acceptable? I didn’t put reflux
Heat with reflux is a better answer.
Shit I wrote prolonged heating. Is that okay?
Anyone knows if dehydration is acceptable instead of elimination for qn 4?
LOL sry I posted the comment before the answers came out. I guess should be ok then
Dehydration is a better answer because water is being removed
dude i spelled adsorption as adsorbed , is it penalised senpai
I am not your “dude”, I am a teacher, so please address me as Mr Lee or Mr Eric. You will not be penalised for your English if the meaning is the same.
For q3aii my sch lecture notes wrote mgo can react very slowly with water to form mg(oh) 2 which is sparingly soluble…..?
Based on how the question is phrased and it is only 1 mark, they are looking for the reaction between CaO and water.
If I wrote both, will it be accepted? 😡
MgO is slightly soluble in water. Both answers can be included. I feel that they are looking for the reaction between CaO and water since it is only 1 mark but it is not wrong to write MgO dissolves in water to form Mg(OH)2.
For q5e if I didn’t write orange k2cr2o7 turns green but wrote which substances will react and what they will form, and add the parts about using co2 gas and orange ppt to distinguish p and q, is that OK? Cause I wrote that the solution without any co2 or orange ppt will therefore be R since its tertiary alcohol. Thank you!
Hey, thanks for the answers 🙂 just want to ask for 3dii, if I didn’t write excess and just wrote ethanolic conc nh3, will I lose the mark? Thanks
You should write “excess” to prevent the product (more nucleophilic than NH3) to react with Compound J.
for q4c, shouldnt we use the fact that the molecule is liquid and thus enthalpy change of vapourisation is not taken into account ?
Good. This reason can also account for the difference in values.
thanks mr lee ! rest early !
Hi Mr Lee, will I be able to get the marks if I converted the value of delta S to Jmol-1, and the volume to dm^3 instead (the conversion was correct)? Thank you very much, really appreciate your efforts in uploading the answers 🙂
The units for delta S should be J K-1 mol-1 if you are converting it. The volume can be in dm3 (make sure the conversion is correct).
hello thank you so much for posting the answers, may i ask for qn 2 iii estimate the value of pv can it be between 8.88×10^5 and 9.26×10^5? (you wrote 9.11×10^5)
It should be roughly in the middle of 8.88×10^5 and 9.26×10^5
For the alpha helix question, is it ok if you mention regular intervals instead of every 4th amino acid?
And is it okay if the mechanism for 4d(I) is correct but I forgot to label is as RDS? Instead I only wrote “slow” above the arrow
every 4th amino acid is important. The question requires to label RDS.
Hi will elimination be accepted for q4a?
It should be accepted.
If I forgot to add the negative sign for 4b and c, will I get marked down heavily?
Enthalpy change of combustion is negative
Hello! May I know how important is “under reflux”? I wrote most of my reactions as just “heat”, because I remember my tutor once saying only strong oxidation of double bonds is reflux absolutely necessary…
According to my Chem teacher, don’t need to write reflux and that write heat is sufficient because reflux is a form of heating or sth
Heat with reflux would be better. Some reactions require reflux because the reactants are volatile and you need to condense the vapours back to the reaction flask in order for the reaction to be complete.
hello, for the entropy change, they didnt specify about the units (and even though yes i know normally it’ll be jmol-1k-1) why cant we put it in kjmol-1k-1 as its not stated that it has to be in standard units?
my answer is in kJ mol-1 K-1. Both units are okay, make sure your conversation is correct.
hi for 3ci is lysine ionic as well?
Lysine is positively charged, it cannot bind to calcium ions which are also positively charged
so if I included lysine, the whole thing is considered wrong?
Marks will be deducted. Ionic bonding should be 1 mark.
Ahh the dreaded excess denied :p thanks for uploading the answers 🙂
I doubt so , because they are asking for binding with calcium ion which is positively charged.
You cant have ionic bond with 2 positiveky charged ion…
this is my reasoning but i think mr eric shoukd verify this hehehe
btw for 3dii i wrote h2so4(aq) but i was abit hesitant bcos the nh2 group can be protonated so is that question abit ambiguous?
sry i mean 3eii
i mean 4eiii oops
yeah i did the above then put “add NaOH(aq) carefully” i think i saw it somewhere in a TYS answer key so im not sure.
Then COOH will be deprotonated.
Using H2SO4(aq) and heat under reflux is the best answer. The NH2 group may be protonated but we need to assume it does not.
I wrote limited H2SO4, it’ll be okay right?
I think the main idea is to use H2SO4 and heat. Don’t worry too much about the protonation of NH2
If I use hcl,reflux will it be accepted because my school did say it could too 🙂 thank you
For Q4di if i never write the 3rd step and i link step 3 and 2 together, is it okay?
i dont think so
Hi, just wondering if answers for question 5 will be posted tonight? Haha otherwise I’ll go to bed already 🙂
I am working on it now. Thanks for waiting.
Hi Mr Lee,
I think you overcounted the number of bonds broken, it should be 6 (C – H) instead of 8, as there are only 2 CH3 groups. This results too much energy taken in for bond breaking. Thank you very much for posting the answers! 🙂
There are 8. 2 more C-H bonds in the 5 membered ring
Hi Mr Lee, if i explained qns 2b iv using the ksp values of the two hydroxides, is it acceptable? Thank you!
you need to explain common ion effect
hi if I wrote Ag(nh3)4 instead of Ag(nh3)2 will I get penalized even though my explanation is correct?
The formula is required by the question, you need to write the correct formula to get full credit
Hello thanks for the answers really appreciate it. For q4c i was careless and didn’t times 100/80 for the energy of experimental entaphy change of combustion :/ But i did explain the reason for the difference in values. Would i still get at least 2 marks?
Explaining consist of just 1 mark bro…
If units write wrong for 3biii, will i be penalised?
Yes, mistakes will be penalised
Hi but if i got the answer right but i put mol3dm^-6? It is stil -1 mark?
Units wrong, -1 mark
For qn 4c my methods are all correct but I used the wrong units. I used KJ instead of J in the begining to find heat change. Will they penalize the whole qn? 🙁
Some marks will be deducted. Take note of the careless mistakes
Thank you 🙂
Can I ask roughly how much marks will I lose??
Hi!Thanks for uploading!For elucidation is the structure worth 4 or 6 marks? It makes a difference for me! Cos the deduction was almost perfect but I only managed to deduce 2 structure correctly.
Will wait for question 5 patiently! 🙂
Question 5 is uploaded. You should be able to get some marks based on your deductions. There should be more than 10 marking points.
Hi for question 5 last part instead of 2,4DNPH. Tollens is also an acceptable reagent right.
Tollen’s reagent cannot differentiate between ketone and carboxylic acid
For Q5cii can we just mention it has diagonal relationship with magnesium hence similar chemical properties
“Diagonal relationship” is not a reason without explanation. There is such a relationship because atomic radius decreases across the period but increases down the group, and electronegativity increases across the period but decreases down the group.
For question 5e, won’t the acid from acidified potassium dichromate(VI) react with carbonate?
Hi mr lee, for q5e, can i say that ONLY P reacts with acidified potassium dichromate heat to distill to give an aldehyde, that will give silver mirror in tollens reagent?
Yay!! Thanks for finishing the upload ^’^ rest well mr lee
Hi Mr Lee,
For 5aii, if i didnt leave it in 2dp, how many marks will i lose? 1 out of 2?
Yup. Take note of careless mistake!
Thanks for the answers mate! God bless
I believe the answer for Q5(b)(ii) should be metallic bonding, not Van der Waal’s forces as Li donates its valence electrons into the pi-electron cloud, forming part of a “sea of electrons” as in metallic bonding (:
I have reviewed the answer and ionic bonding would be a better answer than metallic bonding since graphite is a non-metal.
can it be VDW?
Ionic bonding is present between Li atoms and layers of carbon atoms in graphite
For qn 5e) can we first use dichromate with immediate distillation to determine the pri alcohol and then use KMnO4 to determine the sec alcohol?
The secondary alcohol will also react with dichromate even with immediate distillation.
Hi Sir! Is it possible to use tollen’s reagent as a second step for 5e since the primary and secondary alcohol have been oxidised to aldehyde and ketone after the first step? 🙂
The primary alcohol is oxidised to carboxylic acid..
If you want that though, you must say WARM WITH IMMEDIATE DISTILLATION for P, otherwise you will not get the mark because P will turn into a Carboxylic Acid.
Hello! For 4f, since the qns only asked us to “describe”, will we be penalized if we didn’t write FRS. I only wrote out the equations
you need to write the name of the mechanism.
hi sir, for the last question’s last part can I use Na metal after refluxing with Cr2O72-?
Na metal will react with the water present, so you are not testing anything.
WOW GREAT THAT DIDN’T CROSS MY MIND AT ALL. i seem to lose a part of my brains during exams. thanks for the hard work sir, sleep early.
can use sodium carbonate right? CO2 produced with the COOH formed with the pri alcohol but no CO2 with the ketone formed from sec alcohol?
acidified K2Cr2O7 already contains acid, so it is pointless to use sodium carbonate
For 3c)i), is electrostatic forces of attraction acceptable instead of ionic bonding?
For 5b)iv), they said suggest a reagent for step 1 but they never required condition. So is it ok to write down just KMnO4?
For 5b)v), is nucleophilic substitution a possible answer? Because the carbonyl carbon is electron deficient.
Thank you very much for your suggested answers btw!
3(c)(i) Electrostatic forces of attraction can refer to metallic bonding as well, unless you write clearly it is between positive Ca2+ and the negative COO- groups. Ionic bonding is still better as learned in one of the 4 types of R group interactions in tertiary structure of protein.
5(b)(iv) No, it is important to write cold here, if it is hot, there will be oxidative cleavage. ALWAYS write reagent and condition!
5(b)(v) It should be accepted
For the elucidation question, if I forgot to put am that AFTER OXIDATION of both P and Q add 2,4-DNPH only wrote add 2,4 DNPH to P and Q, is it wrong?
You need to test the KETONE after oxidation of Q. It is important to write after oxidation.
Thank you!! This was really helpful 🙂
hi sir what if i use DIL h2so4 aq to convert CN to cooh?
dilute H2SO4 = H2SO4(aq)
Hi Mr Lee, thanks for uploading the answers so promptly. For 3(a)(ii), Will I get any credit if I only gave the equation but did not describe it. And for 4(a) step 2, will condensation be accepted as an answer
It is better to describe slightly. I don’t think condensation is correct for this case. There are double bonds being formed, so it is dehydration or elimination
hi,
for Question 5iv, if I put cold kmno4(aq) isit okay
for question 5v, if i put Nucleophilic sub isit okay too?
thank you!
5iv No, acidic/alkaline condition must be there.
5v Yes, it’s okay as it’s Esterification actually.
Cold is the main key concept. Nucleophilic substitution should be accepted.
Hi sir for q5iv) is it wrong if I wrote cold acidified kmno4 instead of alkaline? Thanks!
both acidic and alkaline are possible
Hi, a few queries about the answer scheme posted.
3(a)(ii) Should be discussing about the speed of reaction too, no?
3(b)(iv) While common ion effect is true it does not explain why Ba(OH)2 does not precipitate. I think what’s most accurate is to calculate [OH] and [Mg] and [Ba]. Ultimately [Mg] falls but [OH] rises so the effect on the ionic product is not obvious and needs to be determined?
5(e) Should oxidation be done again with like KMnO4 for the reaction with 24DNPH to be more obvious. If not we’re trying to observe an orange ppt in an orange/green solution.
3(a)(ii) no need.
3(b)(iv) No need to calculate, it is only 2 marks. Most importantly is to explain common ion effect.
5(e) No need. To observe the ppt, we can filter the mixture.
if i explained common ion effect using LCP and said that hence solubility of Mg(OH)2 and Ba(OH)2 decreased (but did not say colour of ppt) will i get full 2 marks? How to tell if Ba(OH)2 will be ppted out or not? I just wrote that solubility of Mg(OH)2 and Ba(OH)2 decreases?
only Mg(OH)2 will be ppted out because it has a lower Ksp. Ba(OH)2 will not be ppted out. Solubility of Ba(OH)2 will not be affected. Colour of ppt is important because the question asks for observation
Hi Mr Lee! Thank you so so much for uploading all the answers to allay our worries and doubts 🙂 really appreciate the teachers on this website for their hard work! For math and gp as well 🙂
Just wanted to ask for questtion 4ci) do they accept serine and tyrosine as well?
Thank you!!
No problem. Maths is uploaded by Mr Teng while GP is uploaded by Ms Chen.
Serine and tyrosine are not accepted because their R groups are not charged.
Thank you Mr Lee for the prompt uploading of answers to allay our worries and fears! It means alot to me because my teachers wont tell me if i got them wrong or right afted exams to prevent me from worrying LOL.
By the way for question 4ci) can i say serine and tyrosine too? Or is it wrong…thank you!!
As i have said, not accepted
Is HMF stabilised by resonance?
Yes, but you don’t need to write this in your answer.
Ok thanks! I wrote it in but not sure if it’s correct. I said DMF stabilised by resonance so more energy needed for bond breaking, therefore combustion is less exothermic.
Hi! For Qn 5 bi) should the bonding be ionic bonding? (No I’m not confused with another part). The Lithium ions and Graphite layers form a graphite intercalation compound :/
Yes it forms an intercalation compound and they are stabilized by charge transfer. However, this is not covered in the syllabus. I have reviewed the answer and I have changed it to ionic bonding as I agree it would be a better answer.
4cii) Can just say that density is similar to magnesium without mentioning similar electronegativity and atomic radius? The electronegativity and radius are not the same but it’s actually the net effect that cancels out right?
Hi i would like to know if i didnt mention electronegativity and similar chemical properties and instead wrote similar ionic radius and charge density and the rest are similar for 5cii will it be ok too? And if i didnt write ‘in the nucleus’ for proton number but i stated number of protons will it be fine too?
hello sir, for 4di), is the mech sn1 or sn2? and for dii), we have to take into consideration the cleavage of the double bond? while i admit that i carelessly left that out, shouldn’t the bond be hard to break because of the aromaticity of the ring?
4(d)(i) you don’t need to say it is SN1 or SN2, but it is SN1. The whole group you can just write as R as stated in the question so you don’t need to consider the double bond. The -OH group is not directly bonded to the five membered ring, it is bonded to another CH2 first, so it is not affected by the aromaticity.
thanks sir for staying up to give us the answers and for answering our questions!
Can I write ionic bond for b(i)? For the explanation I wrote that theres is a great difference in electronegativity between lithium and carbon, causeing electron transfer insead of sharing of electron (in covalent bonds) Thus Li and C forms ionic bonds.
I have reviewed the answer and I agree that ionic bonding is a better answer.
Ohh Thankyou! By the way Mr Lee, what is the explanation for that qsn? Cause they ask you to state the bonding and explain your answer.
I just posted the explanation
Hi i would just like to know for qns 3(a) (iv) why would Ba(OH)2 not precipitate too cause i thought both are saturated solutions so IP > Ksp too? Does saturated solution mean that the concentrations are at maximum solubity level (aka ksp level)?
Mg(OH)2 has a much lower Ksp than Ba(OH)2. So Mg(OH)2 will precipitate first. There is no such thing as simultaneous precipitation.
Is it okay if I said Mg(OH)2 will precipitate first, followed by Ba(OH)2 at a later time because Ksp of Mg(OH)2 is higher than Ba(OH)2?
i mean lower*
Hey Mr Lee if my explanation for the transition metal question is because Pd can exhibit variable oxidation states is it correct?
It can exhibit variable oxidation state because…? Due to incompletely filled 3d orbitals!
For Q5 (b) (i) can it be metallic bonding? The delocalised electrons from graphite act as the “sea” of electrons which form metallic bonds with lithium cations? Thank you!
I understand your point. I am hesitant to write metallic bonding because graphite is still considered a non-metal and in metallic bonding, it is the bonding between the cation and the sea of delocalised electrons in the metal itself, not with its neighbour
Hi, why is dispersion forces wrong?
Hi, a few queries about the answer posted:
1biii) will there be error carry forward if i wrote homogeneous catalyst?
3a) is it ok to write mg(oh)(s) instead of (aq)?
5aiii) if i suggested the elements without writing the nucleon number, will i still be able to get some credits?
Thank you!
1(b)(iii) no
3(a) it dissolves very slightly in water and the question asks about MgO reacting in water
5(a)(iii) no
hi sir,
may i know for qn 5bi , can the answer be Van der Waals? due to the ease of bond breaking and forming as the charged ion move across layers of carbon?
🙂 thanks!
Initially I wrote my answer as van der Waals’ forces of attraction but I realise it is not a good answer because lithium only has 3 electrons, so the electron cloud size is quite small to have significant VDW forces of attraction.
hi can the answers for 3ciii) and 3dii) be hcl(aq), heat? thanks!
Yes, but normally we use dilute H2SO4(aq) and heat for acid hydrolysis.
hello for this part, isnt an important condition for hydrolysis heating over several hours (prolonged period) ?
For 3aiv, is it okay to do the calculations for the new IP of both Mg(OH)2 and Ba(OH)2, then compare with ksp? I still managed to conclude that Mg(OH)2 will be precipitate out via this calculation
Not necessary, it is more important to explain common ion effect
Hi, for question 5b part v, i wrote esterification. Is that acceptable? Thanks in advance
the TYPE of reaction is condensation. The reaction itself is esterification
Hi Mr Lee, for question 5(a)(iii) can X be 3H isotope?
No, the proton number must be conserved. 4 protons on the left, 4 protons must be present on the right. X has to be 3He
Hi.
5 the nuclear reaction part i heard from physics students that it has to conserve number of protons. As a bio student I answered tritium, is it still acceptable given that the question doesn’t really say anythingh?
Q4 for the reagent and conditions for furan i feel that kmno4 should be acceptable too because furan is aromatic
Q5 nucleon number definition was a past year question. A required keywork from cambridge is “total” (number of p and n). Not sure whether still required cause only half a mark
Protons and neutrons have to be conserved, that is the idea behind this question. Tritium is not accepted because one proton will be missing.
K2Cr2O7 is a safer option.
Just be clear that it is both the number of protons and neutrons it would be sufficient.
Hi Mr Lee, for q3a do we need to write the equation of the reactions? Because they wrote describe and for past year papers they usually specify ‘with the aid of an equation’ when it is required.
For q5e if I didn’t write orange k2cr2o7 turns green but wrote which substances will react and what they will form, and add the parts about using co2 gas and orange ppt to distinguish p and q, is that OK? Cause I wrote that the solution without any co2 or orange ppt will therefore be R since it is tertiary alcohol. Thank you!
Who didn’t do the last page :[
Hey :(, i didnt do the last page too!!
hi just want to check. as acidified K2Cr2O7 can be used heat with immediate distillation, Q can produce aldehyde too. is it wrong if I use Tollen reagent? quite worried… 🙁
If aldehyde is produced, then you can use Tollen reagent to confirm P is present.
Hi, for question 4(c) I multiplied by 0.8 instead of dividing. Any method marks?
Marks will be deducted.
For 5ai if i didnt put in nucleus will be it ok? I just say in an atom
And for very last qns in 5 after oxdiation i use anhydrous pcl5 to test for white fumes is it ok?
Thanks ! Appreciate ur answers
Should be okay.
No, anhydrous PCl5 can react with water as well to form the white fumes. Water is present.
Oh no… how many marks will be deducted then?
If that’s your only test, then all 3 marks gone! Sorry! 🙁 (If you had a backup test like what I wrote, you should get at least 1 mark assuming all observations are correct.)
I think using oxidation for first step and writing the correct observations should give you 1 mark?
Yes I think all 3marks will be gone!
Yea i did write the first test and tall abt how the tertiary alcohol wont be oxidised so i will still lose 2 marks?
depending on the marking scheme
Hi, for 5e, do I need to state positive tests for all the 3 alcohols ?
Not really…actually at most 2 tests should be good enough for 3 marks. But then again, if you stated positive tests and observations are all correct, you will get the marks.
Hi Mr Lee
For Q5(e), if after doing the K2Cr2O7 test and describe the observations already, I went on to conduct a second test on fresh samples of P, Q and R, the triiodomethane test. Would that be considered wrong?
triiodomethane test is to test for…? P, Q and R will be negative
Hi! For Q5e is it possible to oxidise all of them first with K2Cr2O7, then add Na2CO3 to identify P, followed by iodoform test to identify R? Thanks!
Iodoform test is negative for R, you need a hydrogen atom to be attached to the carbon containing the -OH group for it to be positive. Na2CO3 will react with the acid that is already present in acidified K2Cr2O7, so no.
Hi mr lee, can I use the method of proportionality constant to find a value for PV?
Yes, as long as the value is close to my suggested value
WAH how you know the marking scheme one sia? Thumbs up!
I don’t. It is based on experience. I am not the marker.
But mr lee, the question specifies to ‘estimate’. So would using a proportionality constant be considered wrong as it is too specific and not a method of estimation?
It is okay, it is a form of estimation
Ok thanks mr lee! Thanks for helping by posting ur suggested answers also 🙂
Thanks for uploading the answers!
For 5(a)(iii), for Y, should there be a negative charge on 7 Li (i.e. 7 Li -) because the total charges for reactants, and products should be the same?
the question focuses on the reaction in the nucleus so electrons are not part of the picture here.
If I label the graph wrongly for q1 how much will I get
Assuming your Ea and Enthalpy, Shape shown correctly with Products and Reactants stated it should be 1 mark.
Hi!!! Just wanna understand,
For 2f(ii) why doesn’t delta H and S changes with temperature?
And thanks for the answers
Mr Lee, what is your general opinion on this paper? Was it considered hard or easy?
Easy. Should be 70+ marks for this paper for an A
It’s an easy paper. My friends all had above 70/80.
Hi, for Q5e is it possible to oxidise all 3 alcohols with K2Cr2O7, followed by adding Na2CO3 to identify P and then iodoform reaction to confirm R? Thanks 🙂
tertiary alcohol cannot be oxidised. Iodoform test is negative for all 3 alcohols. Sodium carbonate cannot be used since K2Cr2O7 already contains acid.
Can I write cobalt as the transition metal? :/
Hi there,
for the last part of q5 is 2,4 dnph even acceptable since it cant work in acidified conditions?
Also, for the mg(oh)2 precipitating out q, what if i use calculations to justify it?
hi thank you for the answer!
Can part 5(b)v. be nucleophilic substitution?
Yes, it’s the same thing to describe Esterification! 🙂
If I drew all the full structural formula for the organic compounds and not in simpler form, will I get penalized?
Full structural formula is better if there are no mistakes
Hi! For the question on the definition of proton number, can i write that it is the number of positively charged subatomic particles?
Number of protons. There is no reason to write anything else.
For q 1biii) can I say alter reaction pathway by lowering EA. hence more particles have energy >= EA
you need to be more specific than this. The question is testing on the mechanism of a heterogeneous catalyst
So I will get 0 marks?
Depending on marking scheme
how did you guys do? like approx how much did you guys get? scared cant get A 🙁
57 🙂
I got around 75 how bout the rest?
Wow! out of 80? I got 78 haha, really upset about the 2 marks I lost.
Same for me. Was quite careless forgot to square root when finding pH and other silly stuff 🙁
73!
around 70
I think I got 98 cause I did 5 🙂
Out of 80 or %?
yes out of 80
So good your A is secured. I only got 60-70
An A I would say for this paper should be around 65 or above.
Hey may i noe if 62% is safe for a B??
an A may require 70/80 considering how easy the paper was.
Is there half mark for chem?
No half marks for marking
For question 5 last part, can I use k2cr207 to distinguish the tertiary alcohol, then carry out elimination of water and oxidative cleavage of Alkenes to the new samples of the remaining 2 alcohols ?
sounds possible
But I don’t remember if I wrote “new samples”… Will I be heavily penalized if I didn’t ?
depending on the marking scheme
Dear Mr Lee, for 4(e)(iii) is it okay to put “catalytic amount of conc. H2SO4” without heat? Because won’t the conc. H2SO4 with heat hydrolyse the esters? Thank you! 🙂
You need heat. The reaction is reversible.
Thanks
Hi Mr Lee, for qns 5 last part I read the qns wrongly and use KMnO4 instead of K2Cr2O7, however for the next step I use 2,4-DNPH to distinguish between the remaining two alcohol. Do you think there will be some marks allocated? Thanks 🙂
Maybe
No I got 73%… Out of 100
for me it depends how cambridge marks, like what key points they are looking for and whether I managed to hit them!
Hi Mr Lee, for question 5(a)(iii), will X: He^2- and Y: Li^- be accepted?
Must include the nucleon number
hi anyone knows whats the approximate mark to secure an A for P1??? Thanks!!
Not too sure but my cher says a safe A would be 80% and the papers r kind of weighted so might need a bit more than 80% if ur other papers havent hit 80%
Hey may i noe if 62% is safe for a B??
70% is needed to secure a B, due to how easy the paper was
Yeah right. Check the SG forums. A more credible person is estimating 70-75. This year’s paper wasn’t particularly harder or easier than previous years, no reason for bell curve to shift so much. Even if want to troll also must be more realistic 🙂
70-75 is for an A?
For qn 3(D)i, why does the aldehyde group not undergo nucleophilic addition for H since the CN- nucleophile is present?
you need HCN to have nucleophilic addition. H must be present to convert the carbonyl group to -OH group.
Hi Mr Lee,
For 1biv, if i only wrote palladium catalyst and gave a wrong explanation, how many marks would be deducted?
For 2b, I did not balance the equation of adding cl2 to hot naoh, is it completely wrong?
For 5aii, if i used 6 and 7 for the isotopic masses instead of 6.015 and 7.016, will i be awarded some method marks? or totally 0?
For 5aiii. if i wrote Lithium for Y but my nucleon number is 8. minus 1 mark?
I am not the person with the mark scheme. It is more important to learn from the mistakes than worrying about your marks
Mr Lee, for 1biii, i did not state its a heterogeneous catalyst but my explanation is correct, minus 1 mark?
Better to write heterogeneous catalyst
Hi Sir, may I know is it possible to write dative bond instead of van der waals? Was thinking Li is 1s^2 2S^1 so it has empty orbitals to accumulate delocalised electrons ?
The answer is ionic bonding. Dative bond is not correct, the concept of lithium having empty orbitals to accumulate delocalised electrons is wrong, it is not a transition metal with incompletely filled 3d orbitals to accept electrons to form a complex.
I think paper 3 average around 65/80, 65 should be safe B
I mean of the overall percentage for both p1,p2 and p3 are ard 62% is it safe for a B?
I got 62 /80 for this paper 3. How much out of 80 did you guys get?
Roughly 50++++
I mean If the overall percentage for both p1,p2 and p3 are ard 62% is it safe for a B?
And how much out of 80 did most of your friends get for this paper 3? If you happen to know of course
>60 but most of my friends are potential A graders
Most of your friends got like high 60 or low 60?
If the overall percentage for both p1,p2 and p3 are ard 62% is it safe for a B?
i would say quite high ard >65 those who got B and above for prelims could hit above 70 easily
Which JC are you and your friends from just wondering?
I’m from one of the lower tier jc
Which one? Can you just say?
MJC
And how much did you get for this paper 3 yourself may I ask? Your friends are really smart btw
I got around 70/80 for this paper. Only didnt know how to do the mechanism in q4 and a few careless here and there.
And just being curious how was your chemistry paper 2? How many marks out of 72 do you think you got? Congrats on your paper 3 btw. Really good
personally i felt paper 2 was harder than paper 3 and i don’t really know how well i did because there is no model answers but i screwed up losing around 10 marks tbh
lost 10m in planning or the actual paper?
Is there a half mark for chem paper 3? Some questions require 2 parts to it
No, it is either 1 or 0
How many marks out of 80 did you guys get? just being curious
I got about 61 🙁
Hi Mr Eric Lee. For the reaction of calcium with oxygen, is it acceptable for me to write ‘calcium burns in oxygen with an intense white flame with a tinge of red at the end ‘?
Can
And how much out of 80 did most of your friends get?
So A grade is roughly more than 70 and B grade is roughly more than 65??
Above 60! I missed out on the last page of question 5
64.. could be lower if cambridge expects some strict keywords to be down for some explanation questions, especially the non-calculation ones. kinda disappointed.
Around 60 what? Above 65? Or?
So to get a A grade the overall percentage for all 3 papers is above 70 and the overall percentage for all 3 papers is above 60??
Just because a few people get >70 doesnt mean you need >70 to get A . There will always be high scorers for every paper.
True. I think above 75 marks is quite safe overall. Also need to take into account spa and mcq which probably will pull marks up 🙂 personally I think paper 3 was harder than paper 2 (excluding planning which is only 5%) so I guess if get above 60 for paper 3 is okay because it’s already 75%
May I know Q1aii), as there is a cis-trans isomerism, does it affects the number of optical isomers? As each cis-trans structure will have its own optical activity.
There is no double bond in the structure so no cis-trans
Hi, cis-trans exists when there is a restricted rotation bond? Correct me if I am wrong. Cyclohexane has restrictes rotation. So aren’t both the Carbon bonded to Propyl Group & Carbon bonded to Hydroxyl Group exists as cis-trans isomers? If cis-trans isomers exists, will it cause additional optical isomers?
Geometric isomerism exist when there’s restricted rotation about a pi bond. Cyclohexane does not have pi bond. Also, the 2 groups attached to each carbon must be different. And I think optical isomer isn’t the same as Geometric isomerism. Optical is the ability to rotate plane polarised light.
Cis-Trans exists when there is restricted rotation on bond, not just pi bond. Cis – Trans may affect the number of optical isomers since each cis isomer has a pair of optical isomers & trans isomer has a pair of optical isomers. What does Mr Lee think?
I am afraid that such question may appear in MCQ to test on isomerism.
cis-trans isomerism is only applicable for double bond. Yes, there can be isomers for the orientation of the isopropyl group and the hydroxy group but that is optical isomerism. If you are talking about, for example, a methyl group attached to a cyclohexane, yes the methyl group can be attached with a black wedged or a dotted line, that is conformation isomer. For A level, cis-trans isomerism must contain double bond and the sp2 carbon must contain different groups.
Hi guys how many marks out of 80 did you guys get for this paper?
62-68
Guys how many marks out of 80 did you guys get for this paper?
hello Mr Lee
2f(ii) why doesn’t delta H and S changes with temperature?
For this question, you just need to use the formula and substitute in the values. Higher temperature does not change the energy profile diagram, it only increases the number of particles having energy greater than activation energy (Boltzmann Distribution Graph)
lol, so many ppl above 70? troll or what
Lol, really think/hope they are trolling
ikr if not im dead hahaha bye bye to A alr LOLOLOL i cfm never hit 70 🙁
Even if they are not trolling should still be okay, probably like top 10% above 70?
Jiayous for paper 1 and learn from our mistakes. Like what we leaRN from stats, those commenting here is not a fair sample size so just look ahead:) and do our best for next paper
Hehehe thank you for the much needed positivity ((:
why do you even hope for an A when you know your standard is only worthy of a C grade
What did you score?
Hi Mr Eric Lee for the reaction of calcium oxide with water. Is it acceptable to say that calcium oxide readily dissolves in water?
So around how many marks out of 80 did you guys and your friends get?
79.
guess you should change your name then huh
Hi teacher, this is not relevant to the chem paper. However, may I ask if the question specificly states for a particular method to be used, but we do not use that method, will we get the answer mark?
No. The question is specific like you said.
73 🙂
Hi Mr Lee, is it possible for you to work out and post H3 chemistry solution? Just had my paper today and it was easier than what i expected but i’m scared of careless mistakes.
sorry, but most of us aren’t around this month. :/