All solutions here are SUGGESTED. Mr. Lee will hold no liability for any errors. Comments are entirely personal opinions.
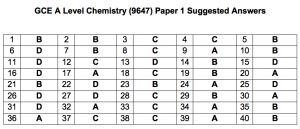
Question 1: B
The element must be from Group IV so the electronic configuration must be either 1s22s22p2 or 1s22s22p63s23p2. The four electrons of highest energy refer to the electrons at shells with higher principal quantum number. The best answer is B.
Question 2: B
Sigma bonds can be formed via head-on overlap between two s orbitals, two p orbitals and between one s and one p orbitals. In hydrocarbons, the carbon atoms have hybrid orbitals consisting of s and p orbitals. Hence, sigma bonds can be formed by either s or p orbitals while the pi bonds can only be formed by side-way overlap between two p orbitals.
Question 3: C
Oxygen atom has 8 electrons. Hydrogen atom has 1 electron. Since hydroxide anion has an extra electron gained, the total number of electrons is 10. Oxygen atom has 8 neutrons while hydrogen atom has no neutrons. Hence, the answer is C.
Question 4: C
A higher charge on Ca2+ would mean that the electrostatic forces of attraction between the cation and the sea of mobile electrons are stronger as compared to Na+. Option D is wrong because the electrons in the calcium ion are not mobile and even if calcium ion has more electrons than sodium ion, it does not explain the stronger metallic bonding. The correct explanation should be calcium metal has more mobile electrons than sodium metal, instead of ion.
Question 5: B
In zwitterion, –NH2 (trigonal pyramidal, 107°) group becomes –NH3+ (tetrahedral, 109.5°).
Question 6: D
Lattice energy is the enthalpy changed when 1 mole of solid ionic compound is formed from its constituent gaseous ions.
Question 7: B
Number of moles of NaOH = 12.5 / 1000 x 0.0500 = 6.25 x 10-4 mol (limiting reagent)
Number of moles of HCl = 25.0 / 1000 x 0.100 = 2.50 x 10-3 mol (in excess)
Number of moles of HCl remaining = 1.875 x 10-3 mol
Concentration of HCl remaining = 1.875 x 10-3 / (37.5 / 1000) = 0.0500 mol dm-3
Question 8: C
CH4 + 2O2 → CO2 + 2H2O
CH4 + 3/2 O2 → CO + 2H2O
9CH4 + 18O2 → 9CO2 + 18H2O
CH4 + 3/2 O2 → CO + 2H2O
Therefore, 10 moles of CH4 react with 19.5 moles of oxygen to produce 9 moles of CO2 and 1 mole of CO (9 : 1 ratio)
Hence, 1 dm3 of CH4 reacts with 1.95 dm3 of oxygen.
Question 9: A
Formation of hydrogen bonds indicates that the reaction is exothermic. ΔH is negative.
The product (1 mole) is more ordered as compared to the reactants (1 mole of CO2 and n moles of H2O). Hence, ΔS is negative.
Question 10: B
Standard conditions means that all concentrations must be 1 mol dm-3.
Question 11: D
Equilibrium concentration for CH3CO2H = C(1 – α)
Equilibrium concentration for CH3CO2– = Cα
Equilibrium concentration for H+ = Cα
Kc = (Cα)2 / C(1 – α) = α2C / (1 – α)
Question 12: C
Increasing the pressure will shift the equilibrium to the right because the forward reaction produces fewer number of moles of gaseous substances. Decreasing the temperature for the exothermic reaction will shift the equilibrium to the right because the forward reaction will release heat energy to counteract the decrease in temperature.
Question 13: D
Total number of moles = 2.0 mol
Number of moles of H2 = 33.3 / 100 x 2.0 = 0.666
Number of moles of CO2 = 33.3 / 100 x 2.0 = 0.666
Percentage of H2O = (100% – 33.3% – 33.3%) / 2 = 16.7%
Number of moles of H2O = 16.7 / 100 x 2.0 = 0.334
Number of moles of CO = 16.7 / 100 x 2.0 = 0.334
Kc = (0.666)2 / (0.334)2 = 3.98 ≈ 4.0
Question 14: B
The reaction is second order with respect to NO. Hence, when concentration decreases by 2, the initial rate will decrease by 4. Hence, x = 6.0 / 4 = 1.5
The reaction is first order with respect to H2. Hence when concentration increases by 2, the initial rate will increase by 2. Hence, y = 1.5 x 2 = 3.0
The reaction is second order with respect to NO. Hence, when the initial rate decreases by (3.0 / 0.75) = 4, the concentration of NO will decrease by 2. Hence, z = 0.5.
Question 15: D
Thermal stability of nitrates increases down Group II due to lower charge density of Group II metal cations as the size of the cations increases.
Question 16: D
Phosphorus exists as P4. Option A is wrong because sulfur can also form two acidic oxides, SO2 and SO3. Option B is wrong because argon should have the highest ionisation energy. Option C is wrong because chlories of silicon (SiCl4) and sulfur (S2Cl2) can react with water to form HCl solution which is acidic.
Question 17: A
Only Option A is correct. Option B is wrong because it is not a precipitate. Option C is wrong because it is a blue precipitate instead of a pale blue solution. Option D is wrong because Cu+ complex should not have any colour because it has completely filled 3d orbitals so no d-d transition is possible.
Question 18: C
Atomic radius should decrease across the period from Mg to P while for melting point, Si has the highest, followed by Al, Mg and P.
Question 19: B
Only dilute sulfuric acid is used to acidify aqueous potassium dichromate(VI). Ethanol can be oxidised to form ethanoic acid.
Question 20: A
PCl5 will react with hydroxyl groups and carboxylic acid groups. The Br atom does not react with PCl5
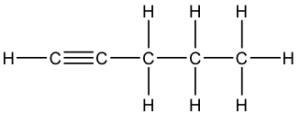
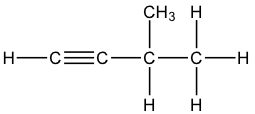
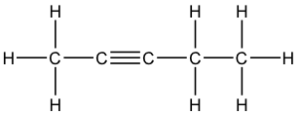
Question 21: B
The 3 structural isomers are:
Question 22: D
Question 23: B
NaBH4 will reduce aldehyde to alcohol but it does not reduce alkene to alkane.
Question 24: A
Molecular formula of citric acid = C6H8O7
Condensation reaction will lose one molecule of water.
Hence, C6H8O7 + C3H6O2 → C9H12O8 + H2O
Hence, option A is the best answer as the structural formula of C2H5CO2H fits the molecular formula of C3H6O2
Question 25: D
Ethanoyl chloride is an acid chloride. It will react with water to produce ethanoic acid and Cl– and the Cl– ion will form a white precipitate (AgCl) with Ag+
Question 26: D
pH 11 is alkaline so the functional groups will be deprotonated.
Question 27: D
Addition of molecular mass of 14 suggests that one oxygen atom is added but two hydrogen atoms are removed. This indicates that primary alcohol (–CH2OH) is converted to carboxylic acid group (–COOH).
Question 28: C
Cold NaOH(aq) is able to react with the acidic phenol group but not with the alcohol group.
Question 29: B
The phenol group is an activating group and is 2,4,6-directing.
Question 30: A
The carbon-carbon double bond will attack the Br–Br first to form a carbocation containing one Br atom.
Question 31: D
Mn2+: [Ar] 3d5 (no paired 3d electrons)
Fe2+: [Ar] 3d6 (one paired 3d electrons)
Co3+: [Ar] 3d6 (one paired 3d electrons)
Question 32: A
Simple covalent compounds have low boiling point. AlBr3 has a simple molecular structure because it has a low boiling point even though it has a metal and non-metal. If AlBr3 is ionic, the boiling point should be 4 digit. Hence, all 3 compounds are covalent.
Question 33: C
The molecules of the gas have mass, so option 1 is wrong.
Question 34: A
Zinc will be oxidised while NH4+ will be reduced. So the overall potential will be +1.50V (energetically feasible). Option 2 and 3 are correct also.
Question 35: B
Rate constants are affected by activation energy and temperature based on Arrhenius equation k = Ae–Ea / RT. Introducing a catalyst will increase both kf and kb equally while increasing the temperature for an exothermic reaction will increase BOTH kb and kf but will increase kb more than kf so equilibrium shifts to the left. Rate constant does not depend on concentration of the reactants.
Question 36: A
Methanal is trigonal planar with respect to carbon and the carbon has an oxidation number of 0 (hydrogen has an oxidation number of +1 while oxygen has an oxidation number of –2). The equation for complete combustion of methanal is:
CH2O + O2 → CO2 + H2O
Hence, 1 mole of oxygen is required for 1 mole of methanal.
Question 37: C
Radicals do not have a lone pair of electrons. They have unpaired electrons and can be formed by homolytic fission of a covalent bond.
Question 38: C
Geometric isomerism is possible if there are two different groups attached to the sp2 carbon in the carbon-carbon double bond. Hence, Y cannot be H and can be Br so that the carbon-carbon double bond with Y can exist as geometric isomers. The carbon-carbon double bonds with Z and X cannot exist as geometric isomers since the groups attached to the sp2 carbon in the carbon-carbon double bond are the same.
Question 39: A
Thymol has a phenol group so the acidic phenol group can react with the alkali present in alkaline KMnO4 via neutralisation. The phenol group can also react with ethanoyl chloride to form an ester via condensation and it can also react with sodium metal to form phenoxide ion.
Question 40: B
Option 1 and 2 have no line of symmetry while option 3 has a line of symmetry. Hence, option 3 is not optically active.
Please do let me know of any mistakes or typing errors that I made while rushing this. Much appreciated and thanks!
[…] Paper 1 […]
Leave a Comment

FIRST!
Thanks Sir for updating the answers!!
What u guys out for qn 9 and 21!!
9)A
21) C
not sure tho!!
9)A
21)B
Why 9 not b 🙁
why 9 not C 🙁
H Bonds are formed, bond forming is exo
21) D. need to consider branched alkynes
Bro, its B. Here’s proof:
https://www.google.com.sg/search?q=c5h8+isomers&oq=c5h8+is&aqs=chrome.1.69i57j0l3.3045j0j4&client=ms-android-asus&sourceid=chrome-mobile&ie=UTF-8#imgrc=OkIjp1O6yHWDKM%3A
Wrong. Even with branched you can’t have that many…it will still be a straight chain for 2 cases due to the triple bond!
need to consider your stupidity and arrogance in thinking you are never wrong.
the number of gaseous particle decrease as co2 becomes aqueous hence entropy negative
But several water molecules 🙁
Entropy is degree of disorderliness…decrease in the number of gaseous particles means delta S is positive (Degree of disorderliness decreases.), that should help choose your answer as A.
Abt how much marks u guys lost ? This year’s was way easier right 🙁
heard A grade for this year is >75%
heard its >80.
the marks range for A usually lower than 70 or more than 70 for past years?
Heard simi heard. You guys arent Cambridge. You wont know how the bell curve is like, the scripts arent even marked yet, stop speculating.
Well said. So sick of all the ‘I heard this’ ‘I heard that’ comments. As if what they heard has a modicum of truth. =. =
Heard its >90
There’s nothing you can do except to pray for the best.
I lost 5 🙁 I’m just hoping that the bellcurve isnt like 80% like high for As. 70-75% still more realistic i think.
I hope so too.. But considering the papers being quite manageable it feels like it’s gonna be more than 75% 😔
37/40. What about u how much u scored?
only got 40…
21 is B… cannot consider cyclic
Yes 21 is changed to B
This is my answer, by no means guaranteed correct
BBCCB
DBCAB
DCDBD
DACBA
BDBAD
DDCBA
DACAB
ACCAC
Q9 A
delta H negative (bond form)
delta G zero (equilibrium)
put into equation, delta S must be negative
Q21 B
the key thing here is to make sure carbon does not contain 5 bonds
pen-1-yne, pen-2-yne and 3-methylbut-1-yne
I think only your last answer wrong, cos option 3 is a meso compound, hence, it does not a net rotation of polarised light. So should be B instead 😛
My answer is B as stated
No20 should be D. -OH can only be nucleophilic sub when there is ZnCl2 catalyst.
No, PCl5 can react with hydroxy groups directly
Why isn’t Q14 A?
second order wrt NO
so if rate *1/4 (3.0–>0.75)
then z must = 1.0 *sqrt1/4 (*1/2) = 0.5
14 is not A because of calculations…try substituting the values in…
What did you all get for qns 2 and 10?
Why aren’t QN 10 and 28 A
For Q10, standard conditions mean everything 1 mol/dm3 except if you are using H2SO4 for H+ concentration. Neutralization will occur as phenol can react with sodium hydroxide
what did u guys put for q38?
for question 1, shouldnt the answer be B?
for question 2, a lot of people are debating if the answer should be D instead because of the phrase “in hydrocarbon”
for question 3, why is the answer not C? (or A)
for question 21, my friends and I only found 3 isomers…
sorry for asking so many questions, thanks for uploading ^^
oops, i meant question 10 instead of 3 (got carried away).
10 is a trick question. Standard condition is always 1moldm-3 for all.
1 and 21 i agree w you
qn 10 everything should be measured under standard conditions
Shouldn’t qn 2 be D since its specifically for hydrocarbons (C and H atoms) only? Hence there will not be any head on overlap by s and p orbitals
Hydrocarbons have C-C too
In hydrocarbons (alkanes, alkenes, arenes and alkynes), there are only carbon-carbon bond n C-H bond.
Between carbon-carbon sigma bond, it is due to overlap of the Cs’ hybrid orbitals, not atomic s or p orbitals.
Between C-H sigma bond, it is due to overlap of the C’s hybrid orbital and H’s atomic s orbital.
Hence, there is no sigma bond formed by overlap of p orbitals in the context of hydrocarbons.
So shouldnt the answer for Qn 2 be D?
Hybrid orbitals are made up of both s and p orbitals, so it is correct to say the sigma bond is formed by overlapping of p orbitals as well. And two p orbitals can form head-on overlap to form sigma bond.
Why is question 35 answer B not D?
Increasing the temperature would increase BOTH kf and kb, for exothermic reactions kb increases more than kf, that’s why equilibrium shifts to the left but both rate constants will increase
Also, can i clarify the bonding in AlBr3? Shouldn’t it be ionic bonding with covalent character? And its only a simple covalent molecule when it dimerises to form Al2Br6?
AlBr3 has a simple molecular structure because of its low boiling point. If it is ionic, the boiling point should be 4 digit
Hi, Internet sources did mentioned that AlBr3 is an ionic bond.
What did y’all get? Just to get a feel? I got 33.
Sigh i got 34
I got 33 too…7 marks lost 🙁 (2 were careless.)
35
37
35
How did you all get 38?! The paper was so hard
40
40. Don’t worry, 33 is a really safe B.
38
Why is Q21 not B?
Q21 is B. I have changed the answer.
33 also
Q1 I think you misread the question, they are not asking which one has the highest energy among the four, but “four electrons w highest energy in its ground state”
Q21 I think you cannot have a carbon with 5 bonds so it can only be pen-1-yne, pen-2-yne and 3-methylbut-1-yne
Q40 shouldn’t it be 2,3 correct? I used r,s convention
Yeah i agree with you…
For question 40, 3 has a line of symmetry while 1 does not.
Q.40
There are 2 chiral carbons in option 1, but because of the plane of symmetry, it’s mirror-image is identical. Such a molecule is said to be a achiral, and does not have optical isomer, nor is it optically active. And these compounds are known as meal compounds.
Option 1 has no plane of symmetry, so only option 3 is not optically active because it has a plane of symmetry
For qn32, AlBr3 is a ionic compound. Google it. Due to the large electronegativity. So answer should be B?
AlBr3 is covalent due to the low boiling point. If it is ionic, the boiling point should be much higher (4 digits)
Google AlBr3, they did provide reasoning why AlBr3 is an ionic compound. But I do believe ionic compounds have a melting point of 4 digit but not boiling point
The ionic bond has high covalent character, you can treat AlBr3 as a simple covalent compound. Boiling point of 265 is considered low and you need to apply the concept that simple covalent compounds have low boiling point
why isnt 1) B? and 2) D?
B for question 1 is correct. Two p orbitals can also have head on overlap to form sigma bond. Also in hydrocarbons, the orbitals are hybrid orbitals involving both s and p orbitals
Hi but the options state s or p, instead of s and p (if its hybrid)
The concept is that two p orbitals can form sigma bond due to head on overlap
But in hydrocarbons, it is impossible for p-orbitals to form a sigma bond.
In the case of alkanes, there are no p orbitals. They are all sp3.
In the case of alkenes, the p-orbitals present will be used to form a pi bond already. There won’t be any sigma bond that can be formed by the p-orbitals.
The same goes for alkynes.
hybrid orbitals contain p orbitals, so it is correct to say p orbitals form sigma bonds.
Do you do answers for H1 chem?
I do not have access to the H1 Chemistry Paper 1. Sorry!
Hi, if you don’t mind, you can drop us an email or send photos of the question to us. 🙂
Hi, can someone explain why Q1 is D? Isn’t that Sulfur? Or did I misunderstand qn ^^
Question 1 is B. I just changed the answer. It was me who misread the question when I was doing the paper while I am on the go
Why is Q39 A? I thought phenol cannot be oxidized and therefore answer should be C?
Side chain oxidation
Hi! But side chain oxidation I thought need to use KMnO4!!
potassium manganate is kmo4 in case you dont know
As in but it’s alkaline!!
it still reacts but the observation is brown ppt formed instead of kmo4 decolourised
Acidic or alkaline KMnO4 both can work for side chain oxidation
I thought only acidified kmno4 will then be able to undergo side chain oxidation? Alkaline conditions work too?
Yes. Also, the alkali will react with the acidic phenol
Why is qn 40 not C? Isn’t option A meso compound so it can’t rotate?
Question 40, option 1 is not meso because there is no line of symmetry
Why isn’t qn 23 D?
NaBH4 cannot reduce alkenes to alkanes
shouldn’t question 40 be C? 1 has a line of symmetry
But the H and CH3 atoms are facing different direction and they cannot rotate, notice the 3-D bond
It’s 3 that has line of symmetry
Where did you draw the line across?
So does 3.
That isn’t a line of symmetry. One goes in and the other comes out. Unlike in option 3
No, only 3 has a line of symmetry
Question 1 should be B. Needs to be in group 4. Option D isnt from group 4, it’s Sulfur, Group 6.
Question 21 should be B as well.
I think
Yes, answers are changed
I think qn 1 should be B and qn 21 also B…
I think the suggested answet for qn1 is wrong. Pretty sure answer is B because of the conditions they gave in the question. Please help to check! Thank you, as always!
Yes, I misread the question. Changed the answer to B.
Hows the bellcurve like guys how did ur friends do?
All of the S and U graders for prelims in my school are able to get >30
From IJC, what talking you, don’t bs pls. Me and my group of U grader friends only WISH we were above 30.
Your statement not valid for ‘all’. Change it to ‘most’ to retain some validity. Although I doubt you are even from IJC. Pic or its not true – that’s how the Internet works. And the onus on you to prove your claims 🙂
Commiserations. Hopefully you will still be able to pass!
Hi! Will you be putting up solutions for H1 chem p1? I have some qns that I’m curious to know what’s the reasoning and workings behind it!
I do not have access to the paper 1 for H1 chemistry. Sorry!
Hi, if you don’t mind, you can drop us an email or send photos of the question to us. 🙂 Mr Lee will be more than willing to help you
Hi! Thanks for the reply! What’s the email address? 😁
http://theculture.sg/2015/11/2015-a-level-h1-chemistry-8872-paper-1-suggested-solutions/
pretty sure qn 2 is D? its a hydrocarbon. C-H cant be formed by p orbitals
C-C bond can be formed too. Anyway two p orbitals can form head-on overlap
Hybridised orbitals considered as using both s and p?
In a way yes. Anyway, two p orbitals can also form head on overlap
yes you must consider C-C is formed by hybridised sp orbitals which means s and p orbitals are involved
I thought question 39 should be C cause only acidified kmno4 can oxidize aromatic carbons to form benzoic acid?
Alkaline KMnO4 can also oxidize
But they didnt state with KMnO4 with HEAT…
Yeah exactly
yeah isn’t there a need to heat it?
The phenol can react with the alkali via neutralisation as well.
The phenol can react with the alkali via neutralisation as well.
I think for qn 39, option 1: alkaline can react with phenol thats why.
I think question 1 should be B and 35 should be D? For 35 if you heat this reaction, forward reaction is exo so by LCP backwards reaction will be favored, Kb will increase but Kf will decrease? Pls correct me if I’m wrong haha. Then for 1 it should be B, which is the config of C, no idea what D is supposed to be ><
And for 32, can anyone explain to me AlBr3 why is correct? :(( in exam I saw like the BP so high then I Sua 😂
BP 265 is not high, if it is ionic the boiling point should be 4 digit. AlCl3 is already covalent, so AlBr3 is also covalent as well since Br has a larger electron cloud size
If you increase temperature, BOTH rate constants would increase, for exothermic reactions, kb will increase more than kf, hence equilibrium shifts to the left
Oh ok I see, thanks Cher!
Got 34 assuming 2 is D
If B then 35
The answer for question 21 should be B right? I asked my chemistry teacher
Yes is B. I changed my answer.
Why is 28 C? I tot neutralisation can only occur at RM temperature
No, neutralization can occur just fine
How about qn 10? Why isn’t the concentration of cl2 halved
Oops saw that 21 is changed thanks!
What would be the “good” / “safe” score to secure A for chem, if paper 2 and 3 get around ~55 each? Is 30 okay? Or will i have to settle for B?
No one will know, so there is no point discussing this
35
why is qn 4 C, since it has diagona;l relationship with Na, i guess both have about same charge density. So D should be the more correct option as it is true that Ca ion has more electrons and so stronger metallic bonding, thus higher melting point.
It is the phrasing of option D. It says calcium ion had more electrons than sodium ion. The electrons in calcium ion are not mobile in the first place so it does not answer the question at all. It should be calcium metal has more electrons than sodium metal. Option C is still the best answer in answering the question
shouldnt qn 4 be D?
The phrasing for option D is wrong. More electrons in calcium ION than in sodium ION does not explain stronger metallic bonding. In metallic bonding, the delocalised mobile electrons in calcium METAL is more than sodium METAL. This is the correct explanation.
3 more hours!!!
why Q4 is C not D? As elemental calcium and sodium have metallic bonding and the number of electrons play a role in the strength in metallic bonding?
I think it’s because number of delocalised electrons is more important and more electrons doesn’t mean more delocalised
Ions have the same number of electrons
yeah.. why isnt it Qn 4 D?
the greater the number of electrons involved in metallic bonding= greater extend of metallic bond??
Thanks!!
i think metallic bond ∝ charge/radius since charge is more significant it should be C
Is the number of valence electrons that contribute to metallic bond, not all electrons
for me my train of thought was that cus metallic bond is dependent on charge density and valence electrons and Ca2+ has only one more valence electron than Na+ so like one electron cant really account for the difference of almost 800 degree haha
hi, sorry but for metals, isnt the main type of bonding metallic instead of vdw (number of electrons)?? Plus, metallic bonding is stronger.
As it has two electrons in its outer quantum shell Calcium can readily release two electrons per atom to the delocalised cloud of electrons that is the basis of the metallic bond. The Sodium atom loses only one electron readily and so the bonding is weaker.
??
Thanks!
Phrasing of option D is wrong because the option talks about the electrons in the calcium ION being more than the electrons in sodium ION. It should be more mobile electrons in calcium METAL. The electrons in the ions are not mobile
It is the phrasing of option D. Calcium ion having more electrons than sodium ion does not have the same meaning as calcium metal having more mobile electrons than sodium metal
Hey just wondering for qn 34, shouldn’t the cell potential be “+1.5v” instead of “1.5v”? Yeah so I chose option C
Without the positive sign, it is still taken as positive. You should have chosen the best answer, which is A
Hi why is qn 35 not D since only catalyst will increase rate in both forward and backwards direction while heating only cause the position of eqm to shift in one direction
hi i think he meant that yes overall the reaction favour backward but when temperature increases it increases the rate of forward and backward just that backward shifts more than forward which causes overall to shift leftwards
Increasing temp will increase rate constant for both but to different extents thus eqm position shifts. But overall, molecules all still move faster regardless
Catalyst increase BOTH kf and kb rate constants by the same amount. Increasing the temperature for an exothermic reaction would increase kb more than kf, that’s why the equilibrium shifts left. Both kf and kb still increase
Hi, could you please upload suggested answers for H1 Chem P1? Thank you!!
I do not have access to H1 Chemistry Paper 1. Sorry!
For Q2 shouldn’t the answer be D since the question specified hydrocarbons and in hydrcarbons, the sigma bonds can only be formed by s and not p orbitals?
The sigma bond is formed between the hybrid orbital of carbon and s orbital of hydrogen OR both hybrid orbitals of carbon in C-C bond. Hybrid orbitals contain both s and p orbitals so it is correct to say that sigma bonds are formed between p orbitals
Why is 20 not B which includes displacement reaction?
Br does not get displaced by PCl5
Please relook qn 23, option B and D looks alike but option B is an alkene.
And B is correct because NaBH4 does not reduce alkene to alkane
Does displacement reaction occur in q20?
Br does not get displaced by PCl5
Q35 should be A. Kc remains unchange since its Kf/Kb. Might be wrong but my textbook says so
kf and kb are only affected by activation energy and temperature.
Hello! For Q2, I thought the answer is D, because hybrid orbitals are not technically s or p orbitals? That’s why I thought only s orbitals (from H) can form sigma bonds, since all C-C head on overlaps are hybrid overlaps.
For Q20, why is the answer not B? Can’t Nu sub also occur between halogens, especially since Br is less electronegative than Cl?
Thanks!
Hi,
I thought for Q4, it’s D cause it has more delocalised electrons so stronger metallic bonding?
The phrasing for option D is wrong. More electrons in calcium ION than in sodium ION does not explain stronger metallic bonding. In metallic bonding, the delocalised mobile electrons in calcium METAL is more than sodium METAL. This is the correct explanation.
Hi for question 38! if Z is H, they can’t form geometric isomerism because there’s 3H atoms????
There is still geometric isomerism the carbon-carbon double bond at Y since Y is Br.
but Y can take a look again
I don’t understand your point. If Y is Br atom, the sp2 carbon with the double bond would have 2 different groups attached to it so geometric isomerism is possible
Got 39 becos of question 2 (my own fault) . But still a score higher than what I expected.
Okay, so arrogant?!?
Perhaps my phrasing was wrg but I didn’t expect to get that high >< sry if it came off as arrogant but I made alot of mistakes in paper 2 and 3. Anyway glad to have learnt something out of this paper 😉 I didn't know sigma bonds were formed as long as there's head on overlapping
Was he being arrogant?
Hello Mr Eric . May I know how many marks at least get for this mcq paper to secure a A?
I got 36/40
No one will know, so there is no point discussing it.
you need 40/40 each mark loss is 0.5mark loss from your overall grade therefore if your overall was 78 40/40 would have gotten you 80
Why is the first statement in qn 34 correct since it is not mentioned that the concentrations of the ions are 1moldm-3 and the gases are at 298k and 1atm?
The E(node) value are given. The “node” symbol indicates that the values are taken at standard conditions.
Isnt the node suggesting the enode value at standard conditions and not the actual value in the cell? I personally saw the question as one that tests whether a student can distinguish the actual E value and the enode value. What is your take on this?
No, the question is not about actual value or E(node) value. It is a normal electrochemistry question.
Why dont you think its about the actual value when the statement says “the cell potential is 1.5V”?
The option is testing you whether you know how to calculate the overall cell potential by treating the E(Zn2+/Zn) cell as oxidation cell and E(NH4+/NH3 + H2) cell as reduction cell
Siao liao. See how it goes for Physics tomorrow.
All the best!
I mean yes we know that theoretically, for Q32 AlBr3 is covalent. However, since the question even stated ‘position of elements present in the periodic table’ and ‘physical properties of the compounds’, and the temperature of AlBr3 is marginally higher than the rest, shouldn’t the answer be B instead? Since we should follow what the question says and not our theoretical knowledge.
265 is still considered low boiling point. If it is ionic, the bp should be 4 digit.
For qn9, isnt formation of hydrogen bonds between CO2 and H2O endothermic since it is unlikely for them to form hydrogen bonds? Like the product is less stable than the reactants hence enthalpy >0?
The question says that hydrogen bonds are formed, and formation of bonds is exothermic.
hi! will u post the answers for the h1 paper? 🙂
I do not have access to H1 Chemistry Paper 1. Sorry!
Can i email u the paper?
http://theculture.sg/2015/11/2015-a-level-h1-chemistry-8872-paper-1-suggested-solutions/
Hi sir I only got 28:((( so many careless mistakes . Is there still a chance of scoring A?
No one will know.
Chill i only gt 26, misread 5 questions qnd gt the tight answer for 3questions but i circled the wrong option…
Like i bet im the only one that got q3 wrong lol
Hello! For question 39, (1) should not be correct 🙂 That is because the reagent is aqueous KMno4, meaning it is dilute. Only conc. KMno4 will cause side chain oxidation to a -COOH 🙂
No need to use concentrated KMnO4 to have side chain oxidation. Secondly, the alkali present in alkaline KMnO4 can also react with the phenol group, which is acidic.
Mr lee, I have another explanation for qn 40 as I feel that ans should be C. My explanation is:
Option 1 is not optically active as the C-H bonds are pointing in opposite directions. Thus each chiral centre does not rotate plane-polarized light in the same direction. As they are in opposite directions, each chiral centre rotates plane-polarized light in opposite directions, thus canceling out each other’s effect on plane-polarized light. Thus as there is no net effect on plane-polarized light, option 1 is not optically active.
Option 3 is optically active however as the chiral carbons are the same, thus each chiral centre rotates plane-polarized light in the same direction. Thus as their effects on plane-polarized light act in the same direction and not oppositely, they do not cancel each other out resulting in a net rotation of plane-polarized light and thus is optically active.
Option 2 is agreed that is optically active so I feel ans should be 2 and 3.
http://chemwiki.ucdavis.edu/@api/deki/files/1572/Cyclic.bmp?size=bestfit&width=621&height=231&revision=1
I believe this will convince u that B is the correct ans?
Sorry but the link that u gave seems to be down?
look again 1 is not optically active you have to look at the bond in its 3D shape because dash bonds superimpose on dash bonds and wedge bonds superimpose on wedge bonds etc
i mean 3 is not optically active
But by looking at the chiral centers, the rotation effects are the same as the chiral centers are the same. How is this wrong?
I don’t think it’s how the atoms are arranged with respect to the chiral carbon that matters, but more of how it is arranged with respect to the entire molecule. 3 has a line of symmetry so it is clearly not optically active
The link works fine for me. If not u can google Meso compunds in ring and see the images
I understand the concept about meso compounds, but looking at the net effect of rotation of plane-polarized light due to each chiral carbon, if the chirality is of the same direction, they would add unto each other?
Mr Lee’s explanation of the line of symmetry still holds. If there is a line of symmetry it means that the two chiral centers actually rotate opposite of each other as the line is sort of like a mirror. Try drawing out the normal diagram of enantiomers.
Alright thanks for the explanation although I still don’t get how my explanation is conceptually flawed but i guess it just doesn’t cut it. Thanks for explaining things to this stupit student here!
It is not the carbon that rotates light, it is the entire molecule.
For molecules with line of symmetry, half of the molecule will rotate light in one way and half in the other way, like if two enatiomers were put together.
I think you are thinking too much into this.
If the molecule is optically active, it must not have a line of symmetry. That is the concept. For Option 1, one group is pointing towards you, the other is pointing away from you. No wonder how you cut the molecule, there is no line of symmetry.
Option 3 has a line of symmetry at the middle, so option 3 is NOT optically active.
Hope this clears up.
Okay thanks all! Maybe I think too much.
How many marks out of 40 did you guys get for this paper?
Can I still get an A for chemistry if I get 32 for MCQ?
Obviously depends on your other papers as well
I lost about 5-7 marks for paper 3.
Then I think can? 70+/80 for paper 3 is quite good imo