All solutions here are SUGGESTED. Mr. Lee will hold no liability for any errors. Comments are entirely personal opinions.
Many thanks to the student who sent us the question paper. 🙂
You can post any questions regarding the answers but please do not ask me how many marks to secure an A for the examination. Nobody will know so there is no point in discussing this.
Showing 14 comments
pingbacks / trackbacks
[…] Paper 1 […]
Leave a Comment

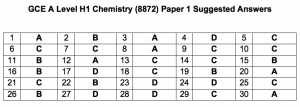
Hi, the suggested answers are not showing. Only the question numbers are showing.
The answers are uploaded. We need time to work out the solutions.
Hi thank you so much for uploading the answers! 🙂 May I know why is qn 8 answer A? It makes sense but what’s the exact concept?
The reason for propane to be liquefied is because under high pressure, the propane molecules are so close together that they can only slide past each other in a disorderly arrangement. Hence, they form a liquid state. This is because propane has more carbon and hydrogen atoms and this is explained by a higher molecular mass as given in Option A.
While Option C seems to answer the question also because more electrons means stronger van der Waals’ forces of attraction, under the high pressure of liquefying propane, the volume of the molecules play a more significant role as compared to the magnitude of the intermolecular forces.
I would think that the size of the molecule is more important and it will better answer the question of why propane can be liquefied more easily.
Hi! may i check if NO2, CO2 and NO can all be removed via catalytic convertor? thanks!
CO2 cannot be removed by catalytic converter. It removes carbon monoxide, oxides of nitrogen and unburnt hydrocarbon
Hi! shoudnt q8 ans be C since another way of putting e qn is why ch4 stay as gas but propane stay as liquid?
The reason for propane to be liquefied is because under high pressure, the propane molecules are so close together that they can only slide past each other in a disorderly arrangement. Hence, they form a liquid state. This is because propane has more carbon and hydrogen atoms and this is explained by a higher molecular mass as given in Option A.
While Option C seems to answer the question also because more electrons means stronger van der Waals’ forces of attraction, under the high pressure of liquefying propane, the volume of the molecules play a more significant role as compared to the magnitude of the intermolecular forces.
I would think that the size of the molecule is more important and it will better answer the question of why propane can be liquefied more easily.
Hi, may I know where we could get a copy of the question sets?
Please erase my comment. I got the question sets from my classmates. Thanks.
hi will you be uploading the h1 chemistry p1 2016? (-:
Nope, sorry. Cos Eric is on vacation and we can’t find a right candidate.
okay thanks anyway! (-: